

Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) is a group of rare diseases that affects small- to medium-sized blood vessels.1,2 Once affected, these blood vessels become inflamed, leading to reduced blood flow and potentially impaired organ function. The exact cause of ANCA-associated vasculitis is currently unknown, but it is believed to be a combination of genetic and environmental factors.1-3
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are the most common types of AAV and manifest as chronic, progressive autoimmune diseases that impact predominantly small-to-medium blood vessels throughout the body.4-6
GPA: granulomatous inflammation affecting blood vessels involving the upper and lower respiratory tracts and the kidneys; other organs can also be affected7,8
MPA: vasculitis often affecting the kidneys, lungs, and skin7
ACTIVE
New, worsening, or persistent clinical signs and/or symptoms attributed to GPA or MPA that are not related to prior damage9
SEVERE
Vasculitis with life- or organ-threatening manifestations9
Approximately 80-90% of patients with ANCA-associated vasculitis present with renal- or other organ-threatening manifestations, which can be considered severe active disease.9,10
Both the humoral pathway and the alternative complement pathway drive inflammatory vascular injury in GPA and MPA.4,5


The interaction between C5a and one of its receptors, C5aR, plays a critical role in amplifying inflammation and vascular injury in GPA and MPA.3-5


Opportunities to further care may start with understanding the humoral and complement mediated pathway of GPA and MPA pathophysiology6,16
ClickTap the icons below to learn more about the potential challenges healthcare professionals face when treating severe active GPA and MPA.
Open conversation between a patient and their HCP is crucial when it comes to treating conditions like severe active GPA or MPA, as each may have different expectations of what successful treatment looks like.27,30 Here we’ve provided some useful topics to help you get the most out of these conversations with your patient.
Your patients may have symptoms that impact their ability to communicate effectively with you, including hearing loss.8 Be sure to consider your patient’s abilities when talking with them, such as facing your patient so they can fully understand you and so that you are both able to read each other’s body language.
Watch a national broadcast Amgen® put together around opportunities to further care in AAV, with a focus on GPA and MPA.
Birmingham Vasculitis Activity Score Resource
The Birmingham Vasculitis Activity Score (BVAS) is a validated tool used to measure disease activity in major studies of vasculitis, including ANCA-associated vasculitis. It captures a broad spectrum of clinical manifestations for new, worsening, or persistent disease across 9 organ systems (1 general and 8 tissue-specific).
The following organizations provide education, support, and resources that may help your patient throughout their treatment journey.

The Vasculitis Foundation supports, inspires, and empowers people and families living with vasculitis through a wide range of education, research, clinical, and awareness initiatives.

NORD (National Organization for Rare Disorders) is a patient advocacy organization committed to the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services.

The American Kidney Fund (AKF) works on behalf of Americans living with and at risk of kidney disease, with programs that support people from prevention through transplant and beyond.
References: 1.Tekin Mİ, Özdal MPC. Acta Medica. 2021;52(4):257-263. 2.Yates M, Watts R. Clin Med (Lond). 2017;17(1):60-64. 3. Shochet L, Holdsworth S, Kitching AR. Front Immunol. 2020;11:525. 4. Kitching AR, Anders H-J, Basu N, et al. Nat Rev Dis Primers. 2020;6(1):71. 5. Al-Hussain T, Hussein MH, Conca W, Al Mana H, Akhtar M. Adv Anat Pathol. 2017;24(4):226-234. 6. Jennette JC, Falk RJ, Bacon PA, et al. Arthritis Rheum 2013;65(1):1-11. doi: 10.1002/art.37715 7. Langford C. Cleve Clin J Med. 2012;79(suppl3):S3-15. 8. Supplement to: Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med. 2021;384(7):599-609. 9. Chung SA, Langford CA, Maz M, et al. Arthritis Rheumatol. 2021;73(8):1366-1383. 10. Lamprecht P, Kerstein A, Klapa S, et al. Front Immunol. 2018;9:1-10. 11. Jennette JC, Nachman PH. Clin J Am Soc Nephrol. 2017;12(10):1680-1691. 12. Mukhtyar CB. In: Watts RA, Scott DGI, eds. Vasculitis in Clinical Practice. Springer; 2010:13-19. 13. Qasim A, Patel JB. In: StatPearls [Internet]. StatPearls Publishing; 2023. Accessed October 3, 2023. https://www.ncbi.nlm.nigh.bov/books/NBK554372/ 14. Hunter RW, Welsh N, Farrah TE, et al. BMJ. 2020;369:m1070. 15. Pagnoux C. Eur J Rheumatol. 2016;3(3):122-133. 16. Chen M, Jayne DRW, Zhao M-H. Nat Rev Nephrol. 2017;13(6):359-367. 17. Jones RB, Ferraro AJ, Chaudhry AN, et al. Arthritis Rheum. 2009;60(7):2156-2168. 18. Winkelstein A. Blood. 1973;41(2):273-284. 19. Eickenberg S, Mickholz E, Jung E, et al. Arthritis Res Ther. 2012;14(3):R110. 20. Ogino MH, Prasanna T. In: StatPearls [Internet]. StatPearls Publishing; 2023. Accessed October 3, 2023. https://www.ncbi.nlm.nigh.bov/books/NBK5553087/ 21. Mohammadi O, Kassim TA. In: StatPearls [Internet]. StatPearls Publishing; 2023. 22. Stone JH, Merkel PA, Spiera R, et al; RAVE-ITN Research Group. N Engl J Med. 2010;363(3):221-232. 23. Terrier B, Pagnoux C, Perrodeau E, et al; French Vasculitis Study Group. Ann Rheum Dis. 2018;77(8):1151-1157. 24. Stone JH, McDowell PJ, Jayne DRW, et al. Semin Arthritis Rheum. 2022;55:152010 25. King C, Harper L. Current Treatment Options in Rheumatology. 2017;3(4):230-243. doi.org/10.1007/s40674-017-0082-y 26. Robson J, Doll H, Suppiah R, et al. Rheumatology (Oxford). 2015;54(3):471-481. 27. Robson JC, Dawson J, Cronholm PF, et al. Patient Relat Outcome Meas. 2018;9:17-34. 28. Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. N Engl J Med. 2021;384(7):599-609. 29. Neumann I. Rheumatology (Oxford). 2020;59(suppl 3):iii60-iii67. 30. Data on file, Amgen; Harris Poll [91973]; 2022. 31. Furuta S, Nakagomi D, Kobayashi Y, et al. JAMA. 2021;325(21):2178-2187.
“Quality of Life” describes how well you’re doing and feeling day-to-day. If your treatment plan is working for you, it can improve your quality of life.
Only about 1/4 of people are satisfied with their medication’s ability to control symptoms while maintaining a good quality of life.*
*According to an online, self-administered survey of 100 patients with GPA or MPA from July 21-August 25, 2022.
Response to induction therapy is variable and many patients do not achieve full clinical remission (defined as a BVAS score of 0 with the patient off glucocorticoids)1
RAVE Clinical Trial: Patients Achieving Full Clinical Remission at 6 Months1


Received one to three pulses of methylprednisolone (1000 mg each) followed by prednisone 1 mg/kg/day.
BVAS = Birmingham Vasculitis Activity Score;
CYC = cyclophosphamide; Q1W = weekly; RTX =
rituximab.
MAINRITSAN-11,2
N = 115 patients with GPA or MPA in complete remission (BVAS = 0) after combined glucocorticoids and “pulse” intravenous CYC
AZA arm (n = 58) received:
2 mg/kg/day until month 12 1.5 mg/kg/day until month 18 1 mg/kg/day until month 22
RTX arm (n = 57) received:
500 mg on days 0 and 14, then months 6, 12, and 18
Prednisone treatment:
Major relapse: reappearance or worsening of disease with BVAS >0 and involvement of at least one major organ, a life-threatening manifestation, or both.
Minor relapse: reappearance or worsening of disease with BVAS >0, not corresponding to a major relapse but requiring mild treatment intensification.
MAINRITSAN-1 Relapse Rates1

AZA = azathioprine; RTX = rituximab. Prednisone dose tapering and the decision to stop prednisone after month 18 were left to each site investigator’s discretion. AZA = azathioprine; BVAS = Birmingham Vasculitis Activity Score; CYC = cyclophosphamide; RTX = rituximab.
Even at lower doses, glucocorticoids can be associated with substantial morbidity1-3
Increased Infections2
Patients receiving glucocorticoid therapy for >6 months have a significantly greater incidence of infections (vs patients not using glucocorticoids after 6 months)*

Patients (N = 147) enrolled in the ANCA disease registry of the GDCN, with an initial diagnosis between 2000 and 2009, and remission on or off therapy attained for at least 1 month.
BMI = body mass index; GDCN = Glomerular Disease Collaborative Network.
Glucocorticoid Toxicity Index (GTI) Assesses Glucocorticoid Toxicity That Can Occur Across Multiple Organ Systems Over Time4

BMI = body mass index.
Renal impairment is common with many patients1,2

GFR = glomerular filtration rate; LTFU = long-term follow-up.
QoL in Patients With AAV vs the General Population2,3
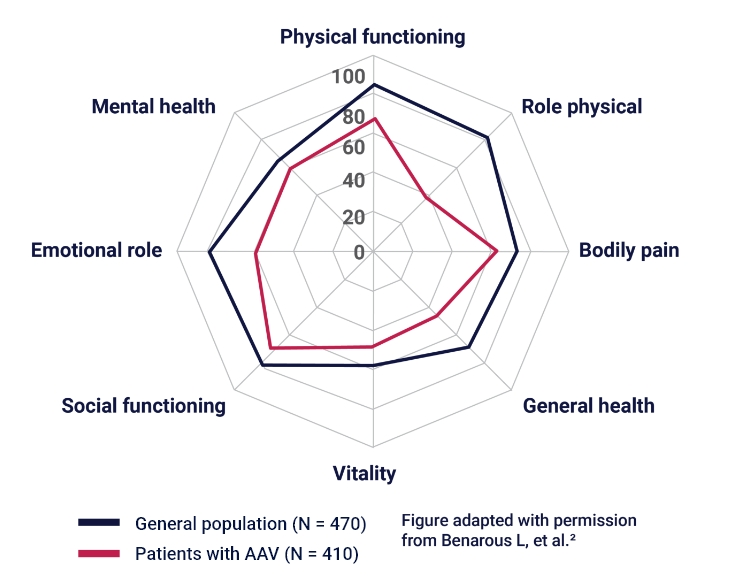

AAV = ANCA-associated vasculitis; QoL = quality of life.
The link you selected will take you away from ANCA101.com to a website that is not owned or controlled by Amgen. Amgen accepts no responsibility for the content of linked sites.
The information contained in this section of the site is intended for US healthcare professionals only.
Click the appropriate button below.
This link goes to the website of a treatment option for adults with severe active GPA or MPA.
The link goes to the website of a treatment option for severe active GPA or MPA. The information contained in this site is intended for U.S. healthcare professionals only.